OVERVIEW
It is well known that lubricants have a finite life. Understanding the lubricant makeup is important, as some chemical and physical aspects can significantly shorten the lifespan of lubricants. These aspects are known as degradation mechanisms and include oxidation, thermal breakdown, microdieseling, additive depletion, electrostatic spark discharge, and contamination. Implementing a trusted oil analysis program can help mitigate degradation mechanisms by employing detection methods to help extend the life of the lubricant.
Oxidation
Oxidation is one of the most common mechanism of lubricant degradation. Oxidation is the reaction between the oil (primarily its base stock) and the oxygen that is found in the atmosphere. The rate of oxidation is highly dependent on the temperature and the period of time the oil is in use. For instance, every 10°C rise in temperature above 70°C, the life of the oil is effectively halved. There are certain factors which catalyse this reaction, including air, water, and wear metals and cause the rate of oxidation to react.
Oxidation generally leads to increased viscosity, acidity, and formation of sludge and varnish. Increased viscosity can cause problems such as increased energy consumption, inability to operate as a coolant effectively, and low fluid flow on start-up that will lead to increased wear. An increase in acidity is another cause of increased wear from acidic corrosion. The formation of sludge and varnish leads to problems of filter plugging.
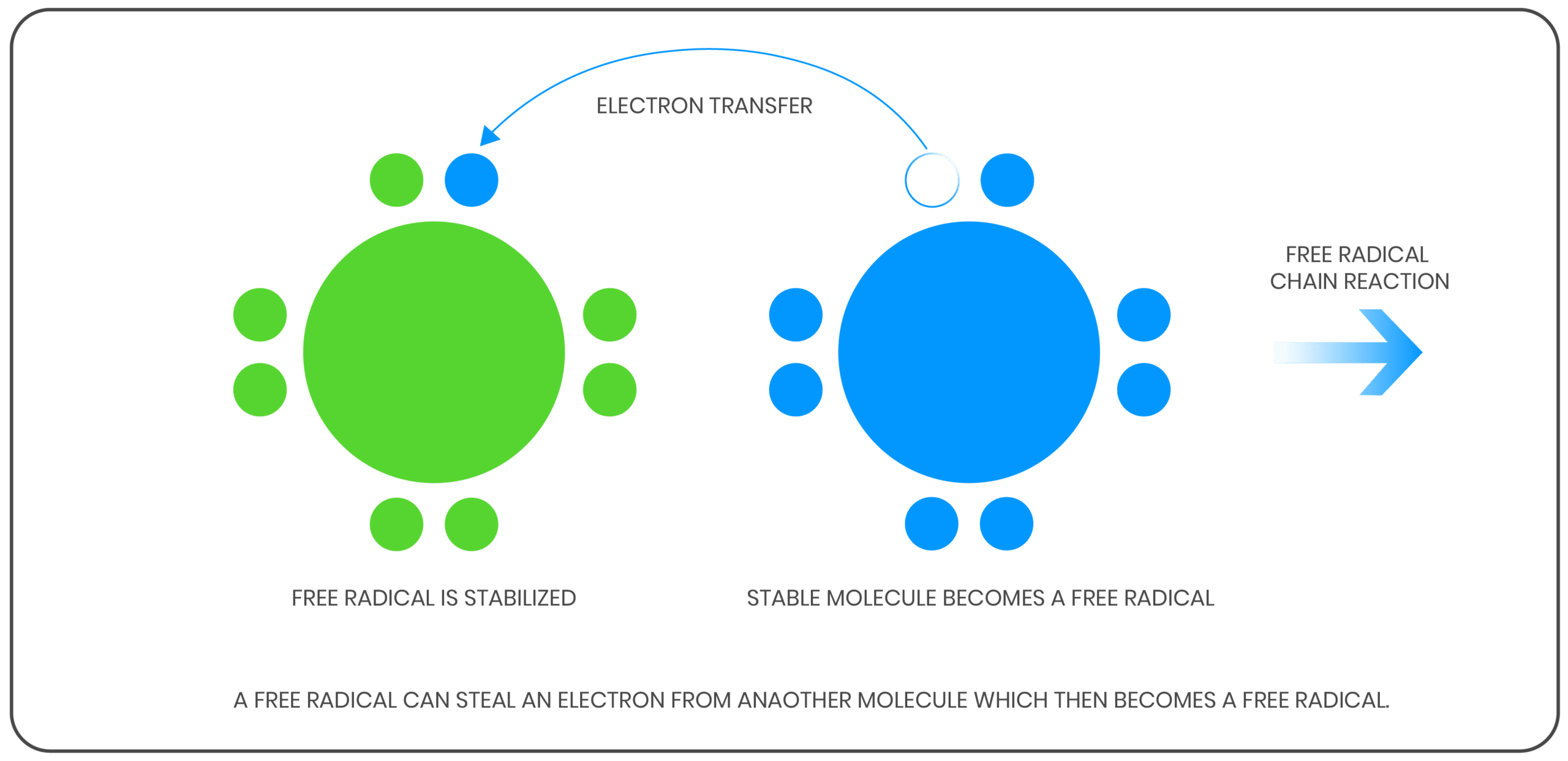
An oxidation reaction goes through a free radical chain mechanism, consisting of three stages.
1. Initiation
2. Propagation
3. Termination
In the initiation reaction, free radicals are formed. Free radicals are atoms or molecules with unpaired electrons, making them highly reactive with hydrocarbon compounds to form hydroperoxides.
In the propagation reaction the hydroperoxides react with the oil, where more free radicals are created. In the presence of catalytic items such as water, wear metals, and high temperatures, peroxides may split and then sustain the reaction. This propagation step continues to be carried out as there is a continuous feed of peroxides to fuel the reaction.
The termination step is due to the presence of the oxidation inhibitor/antioxidant that is an additive in the oil. These inhibitors work by breaking the oxidation chain reaction, decomposing the peroxides that are formed or deactivating the metal surfaces. Antioxidants usually include the following compounds, dithio-phosphates, aromatic amines, and hindered phenols.
There are some detection methods that can be employed to check that the oxidation process is taking place. Not all of the detection techniques need fancy laboratory equipment. The two ways that can indicate that oxidation is taking place without lab equipment is when the oil gives off a foul odour, and then darkening of the oil. Dark oil may not always be oxidized and degraded, but is a possible indication. Oxidized oil would generally have a putrid odour.
In lab detection methods include, total acid number, viscosity, and fourier transform infrared spectroscopy (FTIR), are the main tests that can indicate an oil is oxidising. More advanced tests such as RPVOT, RULER, and MPC can indicate the level of oxidation within the oil.
Thermal Breakdown
Thermal breakdown is another mechanism of lubricant degradation. One of the five main functions of a lubricant is its ability to act as a cooling medium. This means that temperature of the lubricant is a concern. The lubricant can sometimes be heated above its recommended stable temperature and will lead to the loss of lighter hydrocarbon molecules (light ends), resulting in a permanent increase in the oil’s viscosity. It could also lead to some additives being lost and can cause reduced protection/performance/suppression.
Thermal failure generally occurs in the absence of significant amounts of oxygen. One way to detect thermal breakdown is by a change in the colour of the oil, degraded by the oxidation process. Thermal breakdown can also be detected by FTIR by using the nitration peaks as opposed to looking at the oxidation peaks due to the lack of oxygen present. The viscosity of the oil like with oxidation will increase.
Microdieseling
Microdieseling is a process in which an air bubble moves from a low pressure zone to a high pressure zone, and through adiabatic compression it gets heated to very high temperatures. These temperatures can reach up to 1000°C. At these high temperatures, the tiny amount of oil around the bubbles get burned and can lead to carbon by-products, such as sludge and varnish, as well as accelerated oil degradation.
Microdieseling can be controlled if the source of the air bubbles is eliminated. Once again, oil darkening is a sign of a thermal event occurring in a system and microdieseling. FTIR can also give an indication that microdieseling is occurring by looking for by-products such as oxidation in the spectra.
Additive Depletion
Additives are blended into final lubricant products to either enhance base oil properties, suppress base oil properties, or impart new properties to the final product. Most additives are designed to be sacrificial in nature. This makes the monitoring of additives an important part of any oil condition monitoring program. The process can become difficult depending on the additive chemistry, as some times additives that are no longer active will still show up in spectroscopy analysis in the same concentration as when the lubricant was new. The depletion of other additives such as the detergent additive can be determined by measuring the total base number of the oil. Viscosity, total acid number, and infrared analysis also gives an indication of additive depletion.
Electrotstatic Spark Discharge
Oil circulating systems are prone to electrostatic charges by friction caused due to oil flowing along the surfaces of the system. The strength of the static charge depends on the conductivity of the lubricant and the oil flow rate. The lower the conductivity and the higher the flow rate, the greater risk of electrocstatic charging. This charging can accumulate and release a spark between 10 000°C to 20 000°C typically at sharp surfaces and most commonly in mechanical filters. The risk of ESD increases when the oil is formulated with a group II or III base stock, contains no polarising additives, flows through narrow pipes, or contains high proportions of air bubbles.
The conductivity of the oil can be measured in order to prevent damage. Conductivity of the oil is measured by ASTM D2624 (intended primarily for aviation fuels) and has been proven to be effective for lubricants. The electrical conductivity is a measure of the electrostatic chargeability of a substance and is usually it is expressed in pS/m (picosiemens/metre). Generally, if the conductivity is more than 400pS/m at 20°C the risk of ESD is low, and if the conductivity is lower the probability increases.
A lab test, Fourier transform infrared spectroscopy (FTIR) is the most common method to identify electrostatic spark discharge degradation.
Contamination
Contamination of a lubricant generally has three sources, built-in, ingested, and self-generated. Built-in contamination is a contamination that has been left in the component from the original manufacturing process. Ingested contamination is a contamination drawn in from outside the component, for example water, air, dirt, and coolant. Self-generated refers to a contamination that is generated within the component such as wear metals and soot. All of these contaminants have the potential to accelerate the degradation of the lubricant.
Solid particles are the most common particles found in an oil. There a few different ways that these particles are generated, including abrasive, adhesive, and erosive wear due to fatigue. Wear particles that are equal to or slightly larger than the clearance space are the most damaging. Wear particles also form a chain reaction in that these particles become work-hardened, meaning they are harder than the original surface. If not removed by proper filtration, this can cause additional wear.
Water and air can provide a large amount of oxidation potential for reaction with the oil. The most common result of water contamination is rust. Water can also cause certain additives such as ZDDP to become unstable, thereby rendering the anti-oxidant and anti-wear properties that this additive provides null. In colder environments water can cause a lack of lubrication due to freezing of the water. Air is found in four phases in oil; free air, dissolved air, entrained air, and foam. Air contamination has the propensity to cause problems such as pump cavitation and microdieseling.The main oil analysis tests run to detect contamination are elemental spectroscopy, infrared analysis, particle quantification, moisture, fuel dilution, and particle counting.
In conclusion, degradation mechanisms can significantly reduce the performance of a lubricant. Implementing a trusted oil analysis program can utilize detection methods to help mitigate degradation mechanisms to extend the life of the lubricant. A healthy lubricant will protect equipment and will keep your critical asset at peak performance.
Written By:
Deepak Deepnarian
Lab Manager
Bureau Veritas - Oil Condition Monitoring